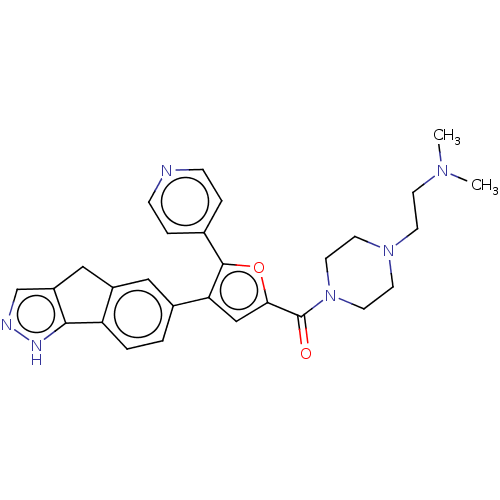
BDBM50482729 CHEMBL1254315
SMILES CN(C)CCN1CCN(CC1)C(=O)c1cc(c(o1)-c1ccncc1)-c1ccc-2c(Cc3cn[nH]c-23)c1
InChI Key InChIKey=ODARJJPHVIUCAP-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50482729
Found 2 hits for monomerid = 50482729
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of human full-length B-Raf V600E mutant expressed in baculovirus infected Sf9 cells after 45 mins by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 740nMAssay Description:Inhibition of B-Raf V600E mutant-mediated ERK1/2 phosphorylation in human WM266.4 cells after 6 hrsMore data for this Ligand-Target Pair