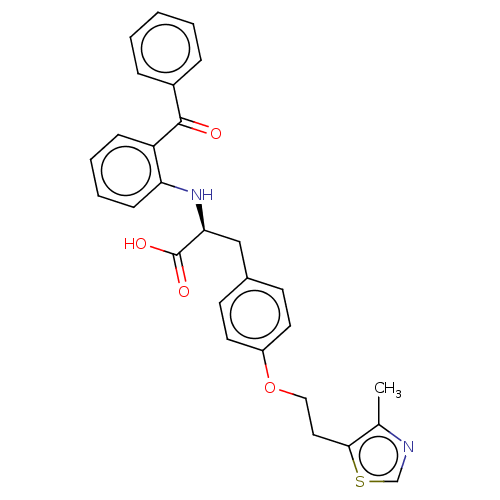
BDBM50471997 CHEMBL343459
SMILES Cc1ncsc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1
InChI Key InChIKey=RLEAWABYZLHNEG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50471997
Found 4 hits for monomerid = 50471997
TargetPeroxisome proliferator-activated receptor gamma(Human)
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataEC50: 759nMAssay Description:Ability to promote differentiation of C3H10T1/2 stem cells to adipocytes using lipogenesis assay mediated through activation of Peroxisome proliferat...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataEC50: 324nMAssay Description:Tested functionally in vitro for inducing 50% of the maximum alkaline phosphate activity (Transactivation) against Peroxisome proliferator activated ...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataKi: 141nMAssay Description:Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataKi: 141nMAssay Description:Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair