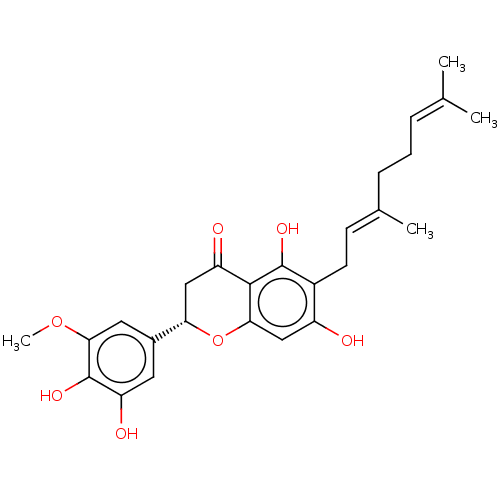
BDBM50468223 CHEMBL463675
SMILES [#6]-[#8]-c1cc(cc(-[#8])c1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc2-[#8]-1
InChI Key InChIKey=MXDFYKXJFUMABT-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50468223
Found 3 hits for monomerid = 50468223
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of ram seminal vesicle COX1 assessed as reduction in PGE2 formation pre-incubated for 5 mins before arachidonic acid addition and measured...More data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+4nMAssay Description:Inhibition of human recombinant COX2 assessed as reduction in PGE2 formation pre-incubated for 5 mins before arachidonic acid addition and measured a...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Human)
The Czech Academy of Sciences
Curated by ChEMBL
The Czech Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of human recombinant 5-LOX assessed as reduction in leukotriene B4 production pre-incubated for 10 mins before arachidonic acid addition a...More data for this Ligand-Target Pair