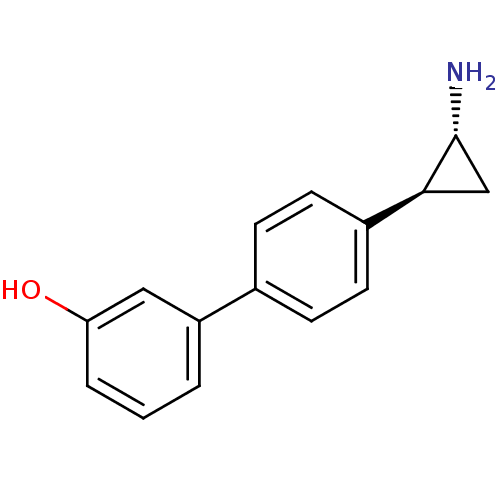
BDBM50445349 CHEMBL3104261::US9676701, 63 Enantiomers of 4′-((trans)-2-aminocyclopropyl)biphenyl-3-ol hydrochloride
SMILES N[C@@H]1C[C@H]1c1ccc(cc1)-c1cccc(O)c1
InChI Key InChIKey=DSOJSZXQRJGBCW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50445349
Found 8 hits for monomerid = 50445349
Affinity DataIC50: 20nMAssay Description:Inhibition of human recombinant LSD1 using dimethylated H3K4 peptide as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.05E+4nMAssay Description:Inhibition of human recombinant LSD1 by horseradish peroxidase-coupled fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 50nM ΔG°: -9.95kcal/molepH: 7.4 T: 2°CAssay Description:Briefly, a fixed amount of LSD1 was incubated on ice for 15 minutes, in the absence and/or in the presence of various concentrations of inhibitor (e....More data for this Ligand-Target Pair
Affinity DataKi: 50nM ΔG°: -9.95kcal/molepH: 7.4 T: 2°CAssay Description:Briefly, a fixed amount of LSD1 was incubated on ice for 15 minutes, in the absence and/or in the presence of various concentrations of inhibitor (e....More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMpH: 7.5Assay Description:Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMpH: 7.5Assay Description:Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMpH: 7.5Assay Description:Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMpH: 7.5Assay Description:Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5...More data for this Ligand-Target Pair