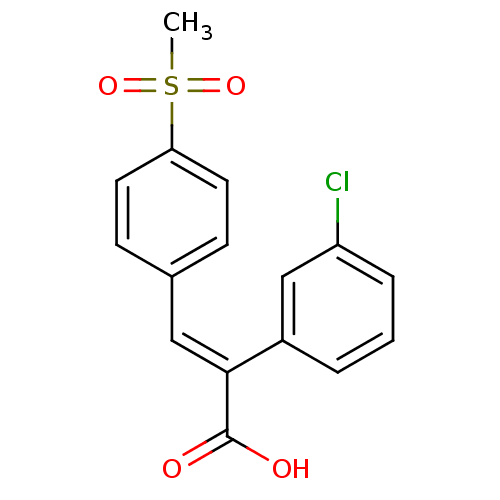
BDBM50429329 CHEMBL2334936
SMILES CS(=O)(=O)c1ccc(\C=C(\C(O)=O)c2cccc(Cl)c2)cc1
InChI Key InChIKey=XZRPHTPUMIPYBH-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50429329
Found 3 hits for monomerid = 50429329
Affinity DataIC50: 2.31E+4nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+4nMAssay Description:Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome...More data for this Ligand-Target Pair
Affinity DataIC50: 7.67E+4nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysisMore data for this Ligand-Target Pair