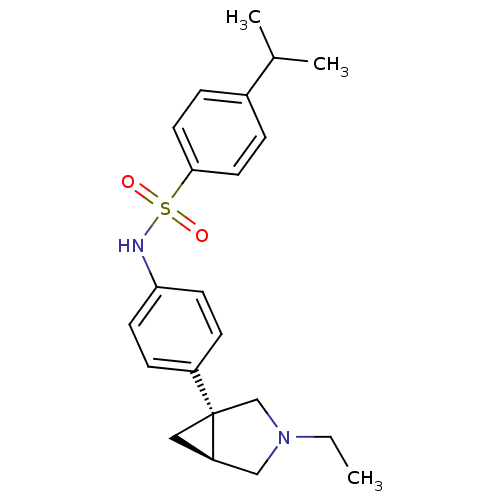
BDBM50416715 CHEMBL1224697
SMILES CCN1C[C@H]2C[C@]2(C1)c1ccc(NS(=O)(=O)c2ccc(cc2)C(C)C)cc1
InChI Key InChIKey=RTBICRVBMOJALC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50416715
Found 3 hits for monomerid = 50416715
Affinity DataIC50: 1.26E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataKi: 7.94nMAssay Description:Antagonist activity at dopamine D3 receptor by GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Antagonist activity at dopamine D2 receptor by GTPgammaS binding assayMore data for this Ligand-Target Pair