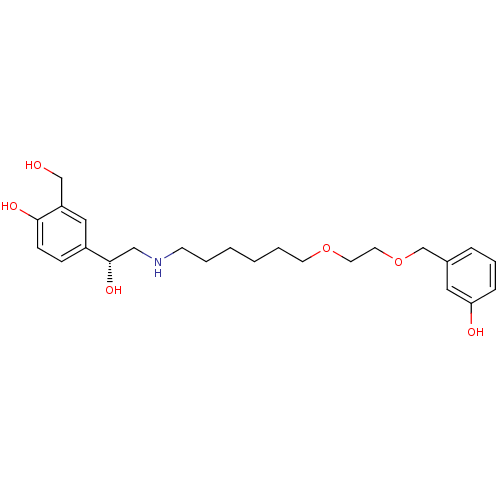
BDBM50416066 CHEMBL1086536
SMILES OCc1cc(ccc1O)[C@@H](O)CNCCCCCCOCCOCc1cccc(O)c1
InChI Key InChIKey=ZXQRIBVIRUROHJ-UHFFFAOYSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50416066
Found 3 hits for monomerid = 50416066
Affinity DataEC50: 0.794nMAssay Description:Agonist activity at human recombinant beta2 adrenergic receptor expressed in CHO cells assessed as induction of intracellular cAMP accumulation after...More data for this Ligand-Target Pair
Affinity DataEC50: 32nMAssay Description:Agonist activity at human recombinant beta3 adrenergic receptor expressed in CHO cells assessed as induction of intracellular cAMP accumulation after...More data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Agonist activity at human recombinant beta-1 adrenergic receptor expressed in CHO cells assessed as induction of intracellular cAMP accumulation afte...More data for this Ligand-Target Pair