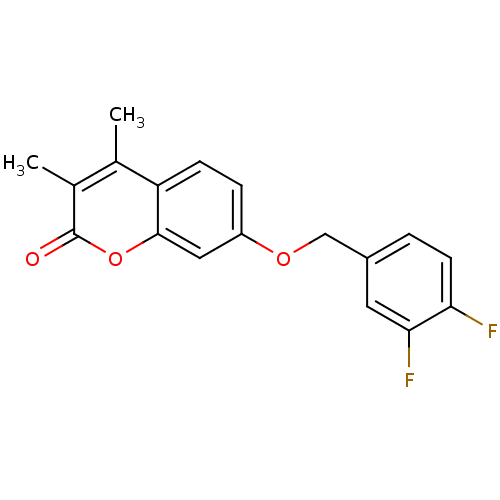
BDBM50409078 CHEMBL325761
SMILES Cc1c(C)c(=O)oc2cc(OCc3ccc(F)c(F)c3)ccc12
InChI Key InChIKey=OAIPPRYRUKGVPD-UHFFFAOYSA-N
Data 11 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50409078
Found 11 hits for monomerid = 50409078
Affinity DataIC50: 1.10nMAssay Description:Inhibitory activity against monoamine oxidase BMore data for this Ligand-Target Pair
Affinity DataIC50: 123nMAssay Description:Inhibitory activity against monoamine oxidase AMore data for this Ligand-Target Pair
Affinity DataIC50: 123nMAssay Description:Inhibitory effect on monoamine oxidase A, SD on IC50 values < 10%More data for this Ligand-Target Pair
Affinity DataIC50: 1.15nMAssay Description:Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10%More data for this Ligand-Target Pair
Affinity DataIC50: 123nMAssay Description:Inhibition of rat brain mitochondrial MAOA using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant human MAO-A using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of MAO-B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Competitive inhibition of rat brain MAO-BMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Human)
Babu Banarasi Das Northern India Institute of Technology
Curated by ChEMBL
Babu Banarasi Das Northern India Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate incubated for 5 mins by DTNB reagent based Ellman's methodMore data for this Ligand-Target Pair