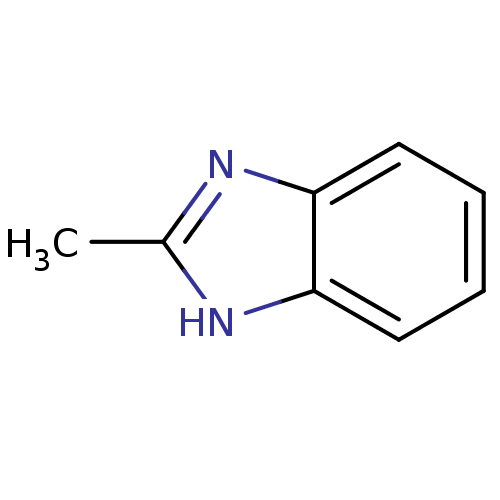
BDBM50404850 CHEMBL309135
SMILES Cc1[nH]c2ccccc2n1
InChI Key InChIKey=LDZYRENCLPUXAX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50404850
Found 4 hits for monomerid = 50404850
Affinity DataIC50: 6.76E+5nMAssay Description:Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70E+5nMAssay Description:Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+6nMAssay Description:Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.07E+6nMAssay Description:Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats.More data for this Ligand-Target Pair