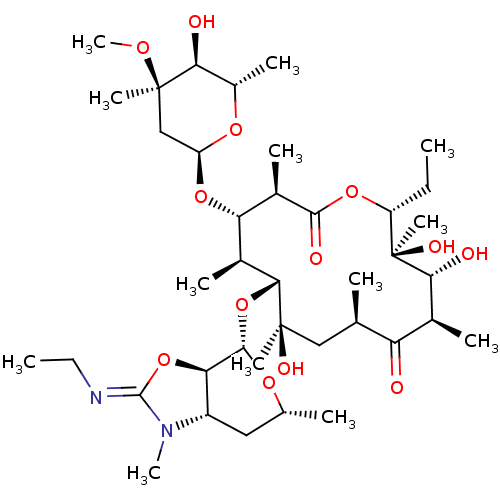
BDBM50393736 CHEMBL2159140
SMILES CC\N=C1/O[C@@H]2[C@H](C[C@@H](C)O[C@H]2O[C@@H]2[C@@H](C)[C@H](O[C@H]3C[C@@](C)(OC)[C@@H](O)[C@H](C)O3)[C@@H](C)C(=O)O[C@H](CC)[C@@](C)(O)[C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@]2(C)O)N1C
InChI Key InChIKey=DLRVDRDTIAJMFM-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50393736
Found 2 hits for monomerid = 50393736
Affinity DataIC50: 500nMAssay Description:Inhibition of human recombinant CYP3A4 using DEF as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-BQ as substrate preincubated for 5 to 10 mins before substrate additionMore data for this Ligand-Target Pair