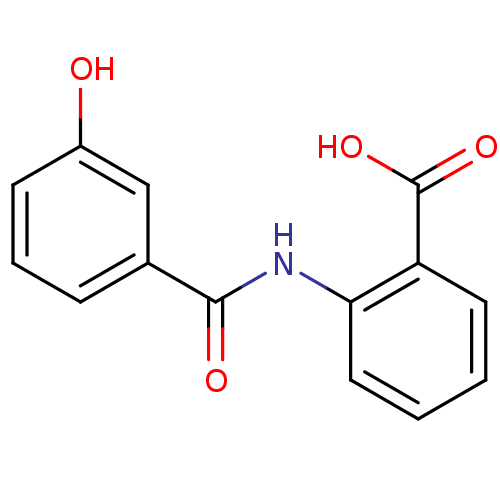
BDBM50390656 CHEMBL2070001
SMILES OC(=O)c1ccccc1NC(=O)c1cccc(O)c1
InChI Key InChIKey=UHYWVEURSKDJJI-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50390656
Found 5 hits for monomerid = 50390656
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant AKR1C1 using S-tetralol as substrate by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.81E+4nMAssay Description:Inhibition of recombinant AKR1C2 using S-tetralol as substrate by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 5.19E+3nMAssay Description:Inhibition of recombinant AKR1C3 using S-tetralol as substrate by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant AKR1C4 using S-tetralol as substrate by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+4nMAssay Description:Inhibition of recombinant AKR1C3 using 1-acenaphthenol as substrate by spectrophotometryMore data for this Ligand-Target Pair