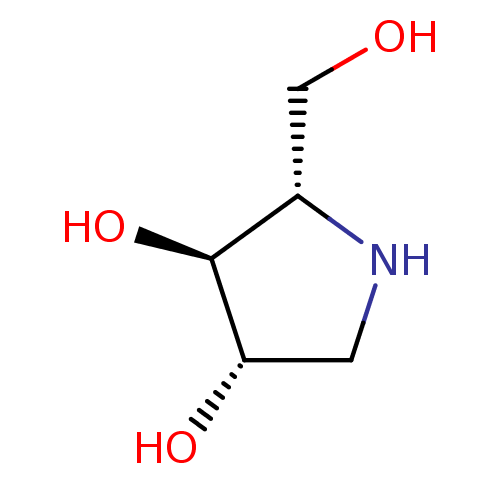
BDBM50375511 CHEMBL406973
SMILES OC[C@@H]1NC[C@H](O)[C@H]1O
InChI Key InChIKey=OQEBIHBLFRADNM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 50375511
Found 19 hits for monomerid = 50375511
Affinity DataIC50: 80nMAssay Description:Inhibition of rat isomaltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human lysosomal acid alpha-glucosidase incubated for 30 mins by fluorescence based spectrophotometryMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha/beta(Mouse)
University of Toyama
Curated by ChEMBL
University of Toyama
Curated by ChEMBL
Affinity DataIC50: 1.63E+5nMAssay Description:Inhibition of yeast alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of rat intestinal sucrase assessed as release of p-nitrophenol using p-nitrophenyl glycoside as substrate by spectrometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of rat intestinal isomaltase assessed as release of p-nitrophenol using p-nitrophenyl glycoside as substrate by spectrometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 930nMAssay Description:Inhibition of rat intestinal maltase assessed as release of D-glucose using maltose as substrate by colorimetric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of Sucrase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 930nMAssay Description:Inhibition of rat intestinal maltase using moltose as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of rat intestinal isomaltase using isomaltase as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of rat intestinal sucrase using sucrose as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4.15E+5nMAssay Description:Inhibition of rat intestinal lactase using lactose as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+4nMAssay Description:Inhibition of rat intestinal trehalaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+5nMAssay Description:Inhibition of porcine kidney trehalaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of amylo-1,6-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of rat sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of rat intestinal maltaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Competitive inhibition of recombinant human lysosomal alpha-glucosidase assessed as inhibition constant by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of green coffee bean alpha-galactosidase using o-nitrophenyl alpha-D-galactopyranoside after 10 to 30 mins by spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.30E+3nMAssay Description:Inhibition of Escherichia coli beta-galactosidase using o-nitrophenyl beta-D-galactopyranoside after 10 to 30 mins by spectrophotometric methodMore data for this Ligand-Target Pair