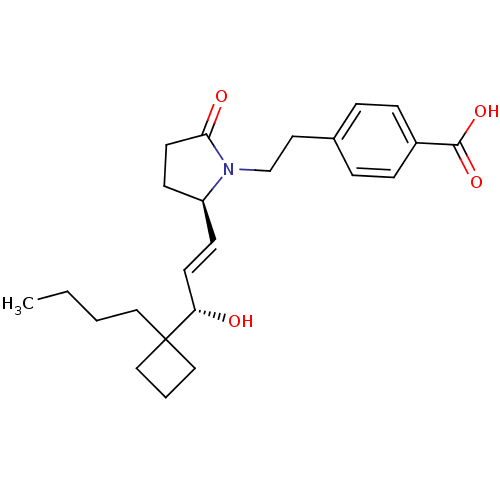
BDBM50373944 CHEMBL272277
SMILES CCCCC1(CCC1)[C@@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O
InChI Key InChIKey=HBUPHLCCOHKMME-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50373944
Found 4 hits for monomerid = 50373944
Affinity DataEC50: 3nMAssay Description:Agonist activity at human EP2 receptor by cAMP assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Agonist activity at human EP4 receptor by cAMP assayMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]PGE4 from human EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Displacement of [3H]PGE2 from human EP2 receptorMore data for this Ligand-Target Pair