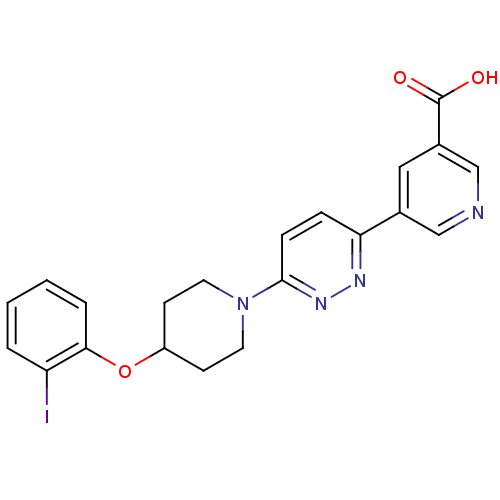
BDBM50364022 CHEMBL1950395
SMILES OC(=O)c1cncc(c1)-c1ccc(nn1)N1CCC(CC1)Oc1ccccc1I
InChI Key InChIKey=RZWTWXUTZXSMLZ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50364022
Found 3 hits for monomerid = 50364022
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of SCD1 in Sprague-Dawley rat hepatocytes assessed as conversion of [14C]oleic acid formation by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.08E+3nMAssay Description:Inhibition of human SCD1 expressed in human HepG2 cells assessed as conversion of [14C]stearic acid to [14C]oleic acidMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of rat SCD1More data for this Ligand-Target Pair