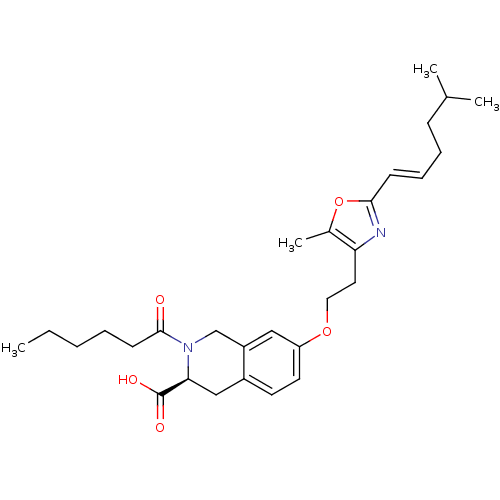
BDBM50361286 CHEMBL1935611
SMILES CCCCCC(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O
InChI Key InChIKey=XGFUSEMJOOJECK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50361286
Found 2 hits for monomerid = 50361286
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Kyoto Pharmaceutical Industries
Curated by ChEMBL
Kyoto Pharmaceutical Industries
Curated by ChEMBL
Affinity DataIC50: 940nMAssay Description:Inhibition of PTB1B using pNPP as substrate after 30 minsMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Kyoto Pharmaceutical Industries
Curated by ChEMBL
Kyoto Pharmaceutical Industries
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair