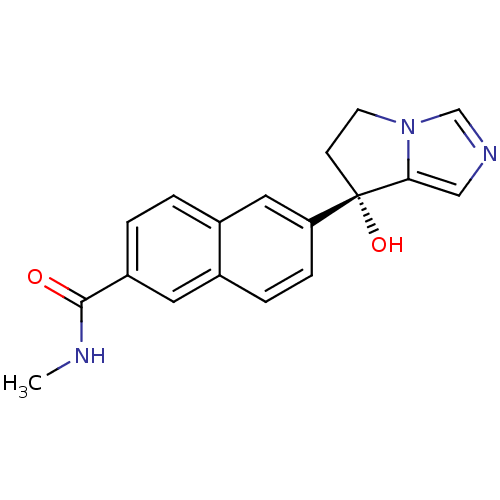
BDBM50358201 CHEMBL1921976::US9611270, orteronel
SMILES CNC(=O)c1ccc2cc(ccc2c1)[C@@]1(O)CCn2cncc12
InChI Key InChIKey=OZPFIJIOIVJZMN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50358201
Found 17 hits for monomerid = 50358201
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C9 (Arg) assessed as Tolbutamide hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Human)
Vanderbilt University School of Medicine
Curated by ChEMBL
Vanderbilt University School of Medicine
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Human)
Vanderbilt University School of Medicine
Curated by ChEMBL
Vanderbilt University School of Medicine
Curated by ChEMBL
Affinity DataKd: 40nMAssay Description:Binding affinity human CYP17A1More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Human)
Vanderbilt University School of Medicine
Curated by ChEMBL
Vanderbilt University School of Medicine
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2E1 assessed as 4-nitrophenol hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2D6 assessed as (+)-bufuralol 1'-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C19 assessed as (S)-mephenytoin 4'-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP3A4 assessed as testosterone 6beta-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C8 assessed as Tolbutamide hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2B6 assessed as ethoxycoumarin O-deethylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP1A2 assessed as 7-ethoxyresorufin O-deethylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Human)
Vanderbilt University School of Medicine
Curated by ChEMBL
Vanderbilt University School of Medicine
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C9 (Arg) assessed as Tolbutamide hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2A6 assessed as coumarin 7-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)