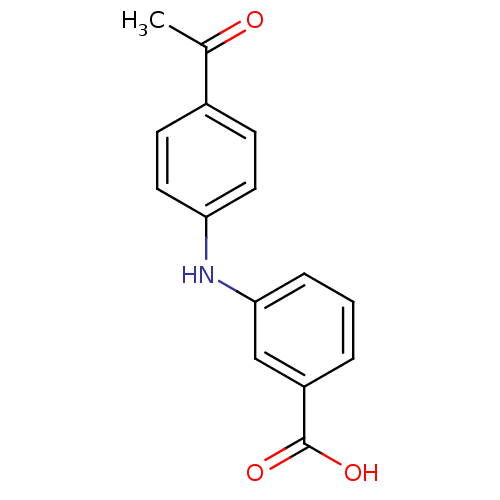
BDBM50337282 3-[N-(4-acetylphenyl)amino]benzoic acid::CHEMBL1682201::US9271961, 6
SMILES CC(=O)c1ccc(Nc2cccc(c2)C(O)=O)cc1
InChI Key InChIKey=WVJCBUXADPESMP-UHFFFAOYSA-N
Data 17 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50337282
Found 17 hits for monomerid = 50337282
Affinity DataIC50: 54nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX2 expressed in baculovirus infected SF-21 cells assessed as formation of PGH2 from PGG2 using arachidonic acid as substrate preincub...More data for this Ligand-Target Pair
Affinity DataIC50: 3.86E+4nMAssay Description:Inhibition of recombinant AKR1B1 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.71E+4nMAssay Description:Inhibition of recombinant AKR1B10 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.95E+4nMAssay Description:Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.57E+4nMAssay Description:Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+4nMAssay Description:Inhibition of recombinant AKR1C1 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of AKR1C2 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.57E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.71E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.86E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair