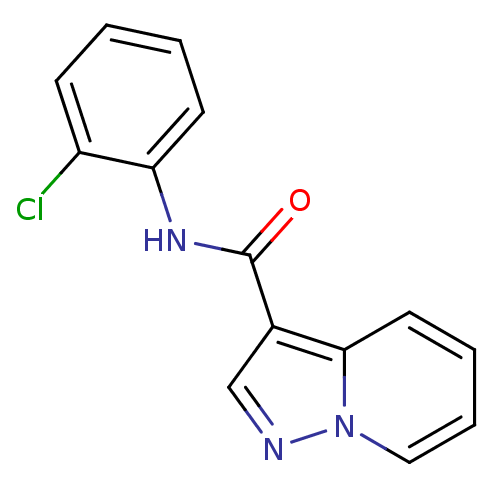
BDBM50311288 CHEMBL1078785::N-(2-chlorophenyl)pyrazolo[1,5-a]pyridine-3-carboxamide
SMILES Clc1ccccc1NC(=O)c1cnn2ccccc12
InChI Key InChIKey=CPQRFZBWMKEHSA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50311288
Found 3 hits for monomerid = 50311288
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 2(Human)
Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Human)
Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of Lck (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)