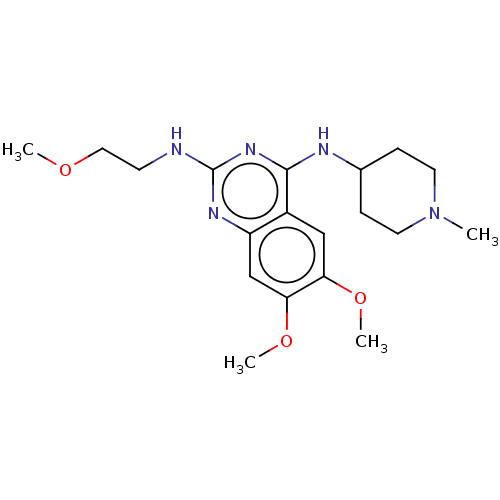
BDBM50268897 CHEMBL4076634
SMILES COCCNc1nc(NC2CCN(C)CC2)c2cc(OC)c(OC)cc2n1
InChI Key InChIKey=KUAVQPWNIWMBPI-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50268897
Found 2 hits for monomerid = 50268897
TargetHistone-lysine N-methyltransferase EHMT2(Human)
Icahn School of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 1.58E+3nMAssay Description:Inhibition of human G9a catalytic domain (913 to 1193 residues) expressed in Escherichia coli BL21 (DE3) using biotinylated H3 (1 to 25 residues) as ...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT1(Human)
Icahn School of Medicine At Mount Sinai
Curated by ChEMBL
Icahn School of Medicine At Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 135nMAssay Description:Inhibition of human GLP catalytic domain (951 to 1235 residues) expressed in Escherichia coli BL21 (DE3) using biotinylated H3 (1 to 25 residues) as ...More data for this Ligand-Target Pair