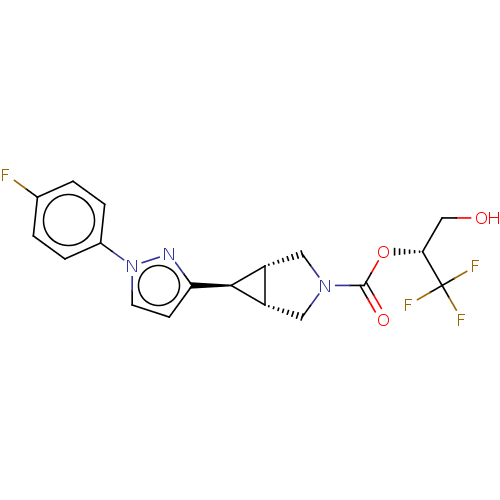
BDBM50255775 CHEMBL4079080
SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1ccn(n1)-c1ccc(F)cc1)C(=O)O[C@H](CO)C(F)(F)F
InChI Key InChIKey=MEDCQBUTCWNKGW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50255775
Found 4 hits for monomerid = 50255775
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of recombinant human FAAH using fluorogenic arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured...More data for this Ligand-Target Pair
Affinity DataEC50: 1.50E+3nMAssay Description:Agonist activity at human CB1 receptor expressed in CHOK1 cells assessed as induction of cAMP after 20 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair