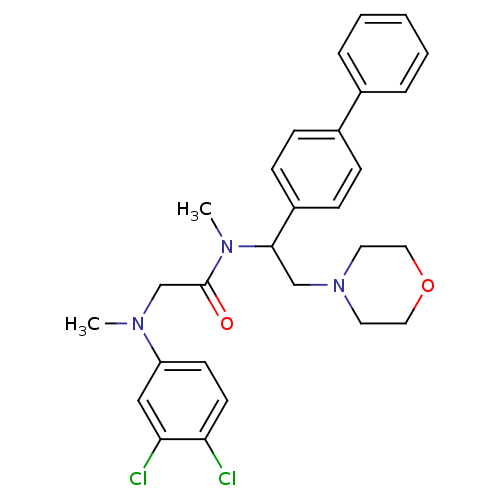
BDBM50243969 CHEMBL452298::N-(1-Biphenyl-4-yl-2-morpholin-4-yl-ethyl)-2-[(3,4-dichloro-phenyl)-methyl-amino]-N-methyl-acetamide
SMILES CN(CC(=O)N(C)C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1
InChI Key InChIKey=ALINQGUROWXGAJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50243969
Found 4 hits for monomerid = 50243969
Affinity DataEC50: 1.60E+3nMAssay Description:Agonist activity at kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin-2 receptorMore data for this Ligand-Target Pair