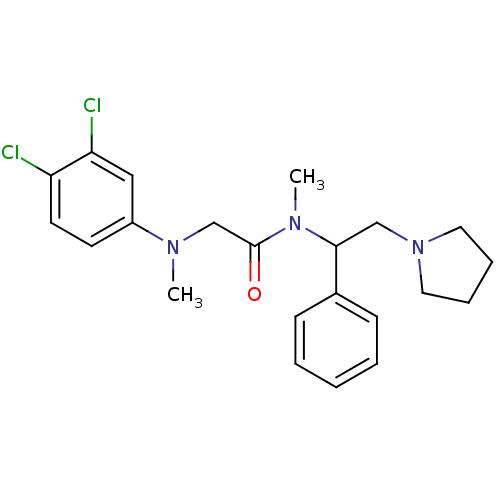
BDBM50243868 (+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-N-methyl-N-(1-phenyl-2-(pyrrolidin-1-yl)ethyl)acetamide::CHEMBL527199
SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1
InChI Key InChIKey=ZAFTWOVROZMXTK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50243868
Found 4 hits for monomerid = 50243868
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Binding affinity to human urotensin-2 receptorMore data for this Ligand-Target Pair