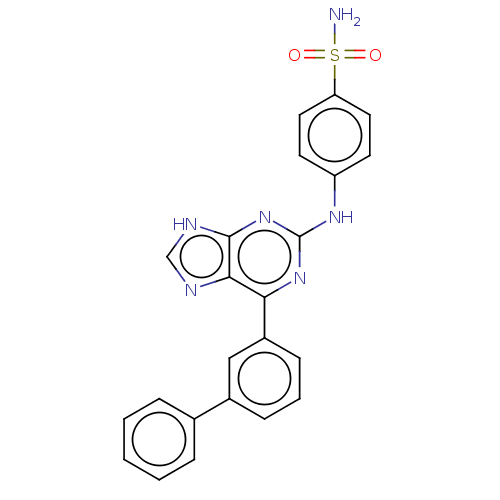
BDBM50235334 CHEMBL4070391
SMILES NS(=O)(=O)c1ccc(Nc2nc(-c3cccc(c3)-c3ccccc3)c3nc[nH]c3n2)cc1
InChI Key InChIKey=FUGRWXRQJGJIER-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50235334
Found 10 hits for monomerid = 50235334
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Human)
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataIC50: 8.60E+4nMAssay Description:Inhibition of recombinant human CDK1/Cyclin B expressed in baculovirus infected Sf9 cells using PKTPKKAKKL-NH2 as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of human GST-tagged CDK2/bovine His-tagged Cyclin AMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of recombinant human full-length C-terminal His6-tagged Cdk9/human full-length Cyclin T expressed in baculovirus infected Sf21 insect cell...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Human)
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of CDK2/cyclin E (unknown origin) using peptide substrate in presence of [gamma33P]ATP by image analyserMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Human)
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of CDK1/cyclin B (unknown origin) using peptide substrate in presence of [gamma33P]ATP by image analyserMore data for this Ligand-Target Pair
TargetCyclin-A2/Cyclin-dependent kinase 2(Human)
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of CDK2/cyclin A2 (unknown origin) using peptide substrate in presence of [gamma33P]ATP by image analyserMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1(Human)
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CDK4/cyclin D1 (unknown origin) using peptide substrate in presence of [gamma33P]ATP by image analyserMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of CDK9/cyclin T1 (unknown origin) using peptide substrate in presence of [gamma33P]ATP by image analyserMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Human)
Newcastle University
Curated by ChEMBL
Newcastle University
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of CDK1/cyclin B1 (unknown origin) using FAM-labeled peptide and ATP as substrate preincubated for 10 mins followed by substrate addition ...More data for this Ligand-Target Pair
TargetCyclin-A2/Cyclin-dependent kinase 2(Human)
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Institute For Organic Syntheses (Vuos)
Curated by ChEMBL
Affinity DataIC50: 543nMAssay Description:Inhibition of CDK2/cyclin A2 (unknown origin) using FAM-labeled peptide and ATP as substrate preincubated for 10 mins followed by substrate addition ...More data for this Ligand-Target Pair