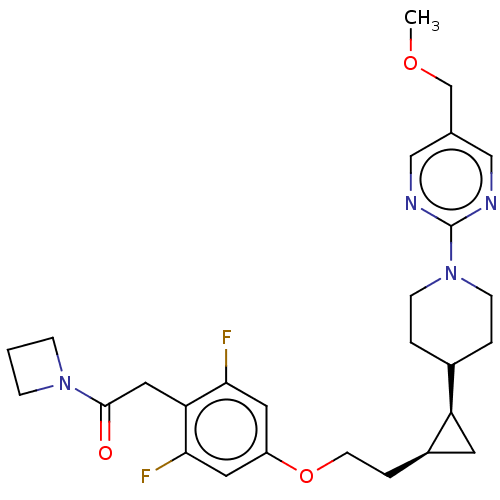
BDBM50232621 CHEMBL4093284
SMILES COCc1cnc(nc1)N1CCC(CC1)[C@H]1C[C@H]1CCOc1cc(F)c(CC(=O)N2CCC2)c(F)c1
InChI Key InChIKey=QWNGDOXELSIKNT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50232621
Found 6 hits for monomerid = 50232621
Affinity DataEC50: 0.800nMAssay Description:Agonist activity at mouse GPR119 expressed in CHO cells co-expressing Galpha15 assessed as increase in intracellular cAMP level after 60 mins by HTRF...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human ERG by PatchXpress methodMore data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human GPR119 expressed in HEK293 cells assessed as increase in intracellular cAMP level after 60 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Activity at human PXRMore data for this Ligand-Target Pair
Affinity DataIC50: 9.10E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair