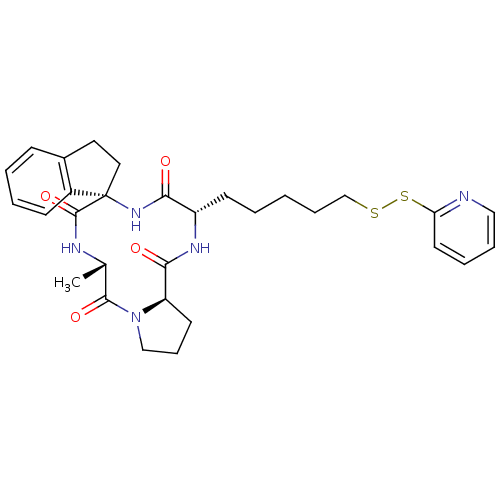
BDBM50222735 CHEMBL390991::cyclo(-L-Am7(S2Py)-D-A1in-L-Ala-D-Pro-)
SMILES C[C@@H]1NC(=O)[C@@]2(CCc3ccccc23)NC(=O)[C@H](CCCCCSSc2ccccn2)NC(=O)[C@H]2CCCN2C1=O
InChI Key InChIKey=ZGCIQVPLYZBUHQ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50222735
Found 3 hits for monomerid = 50222735
TargetHistone deacetylase 6(Mouse)
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of mouse HDAC6 expressed in 293T cellsMore data for this Ligand-Target Pair
TargetHistone deacetylase 1(Human)
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Inhibition of human HDAC1 expressed in 293T cellsMore data for this Ligand-Target Pair
TargetHistone deacetylase 4(Human)
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human HDAC4 expressed in 293T cellsMore data for this Ligand-Target Pair