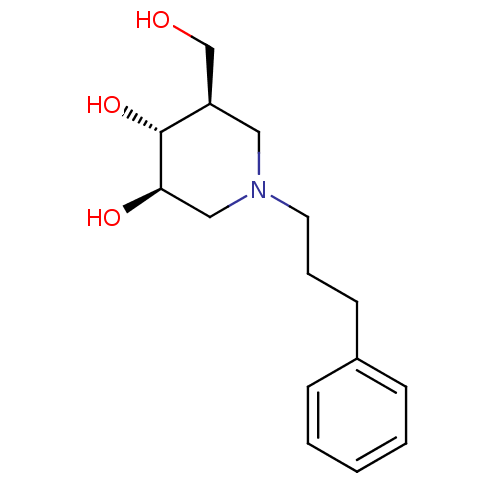
BDBM50194702 (3R,4R,5R)-5-(HYDROXYMETHYL)-1-(3-PHENYLPROPYL)PIPERIDINE-3,4-DIOL::(3R,4R,5R)-5-hydroxymethyl-1-(3-phenylpropyl)-3,4-piperidinediol::CHEMBL213661
SMILES OC[C@H]1CN(CCCc2ccccc2)C[C@@H](O)[C@@H]1O
InChI Key InChIKey=NKARZGURZMIRMA-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50194702
Found 3 hits for monomerid = 50194702
TargetGlycogen phosphorylase, liver form(Rat)
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.22E+3nMAssay Description:Inhibition of phosphorylated form of rat liver glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Human)
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataIC50: 850nMAssay Description:Inhibition of phosphorylated form of pig liver glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Rabbit)
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataIC50: 840nMAssay Description:Inhibition of non-phosphorylated form of rabbit muscle glycogen phosphorylaseMore data for this Ligand-Target Pair