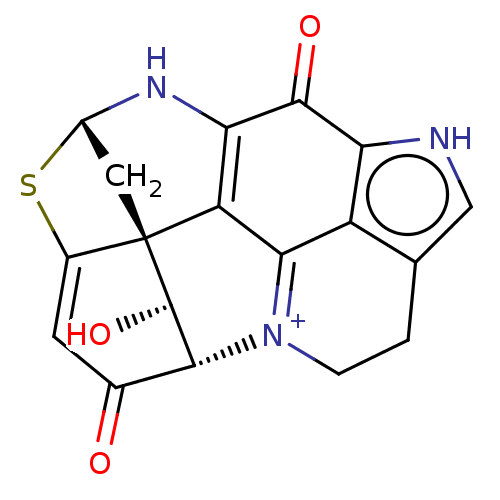
BDBM50190657 CHEMBL1194747
SMILES [H][C@@]12C[C@@]34[C@@H](O)[C@@]([H])(C(=O)C=C3S1)[N+]1=C3c5c(CC1)c[nH]c5C(=O)C(N2)=C43
InChI Key InChIKey=QJMJSLBHDYZDDN-UHFFFAOYSA-O
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50190657
Found 5 hits for monomerid = 50190657
Affinity DataIC50: 730nMAssay Description:Displacement of GST-tagged p300-CH1 domain (323 to 423 residues) from synthetic biotinylated HIF-1alpha C-TAD domain (786 to 826 residues) (unknown o...More data for this Ligand-Target Pair
Affinity DataIC50: 2.57E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured over 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.58E+5nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate measured over 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.31E+5nMAssay Description:Reversible competitive inhibition of equine serum BChE using acetylthiocholine iodide as substrate measured over 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Reversible competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured over 5 mins by Dixon plot analysisMore data for this Ligand-Target Pair