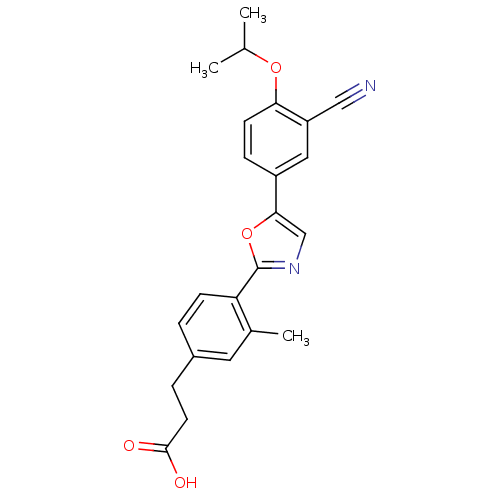
BDBM50186388 3-(4-(5-(3-cyano-4-isopropoxyphenyl)oxazol-2-yl)-3-methylphenyl)propanoic acid::CHEMBL209003
SMILES CC(C)Oc1ccc(cc1C#N)-c1cnc(o1)-c1ccc(CCC(O)=O)cc1C
InChI Key InChIKey=RAOLAYXVJBCRAY-UHFFFAOYSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50186388
Found 3 hits for monomerid = 50186388
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at S1P5 receptor assessed as induction of [35S]GTP-gamma-S bindingMore data for this Ligand-Target Pair
Affinity DataEC50: 5.10nMAssay Description:Agonist activity at S1P1 receptor assessed as induction of [35S]GTP-gamma-S bindingMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at S1P3 receptor assessed as induction of [35S]GTP-gamma-S bindingMore data for this Ligand-Target Pair