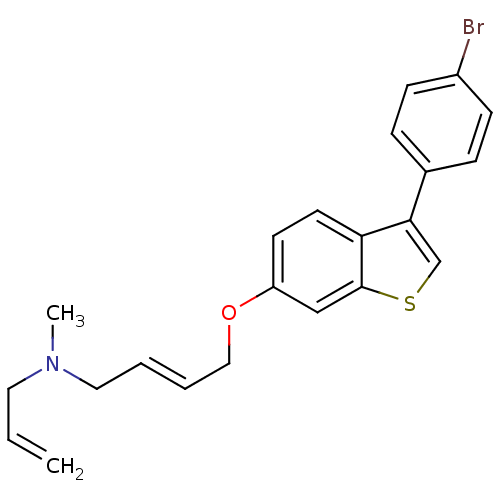
BDBM50128064 (E)-N-allyl-4-(3-(4-bromophenyl)benzo[b]thiophen-6-yloxy)-N-methylbut-2-en-1-aminium::Allyl-{4-[3-(4-bromo-phenyl)-benzo[b]thiophen-6-yloxy]-but-2-enyl}-methyl-amine::CHEMBL65296::N-allyl-4-(3-(4-bromophenyl)benzo[b]thiophen-6-yloxy)-N-methylbut-2-en-1-aminium
SMILES CN(CC=C)C\C=C\COc1ccc2c(csc2c1)-c1ccc(Br)cc1
InChI Key InChIKey=LLPVLKAPTAKNIR-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50128064
Found 4 hits for monomerid = 50128064
TargetSqualene--hopene cyclase(Alicyclobacillus acidocaldarius)
Albert-Ludwigs-University of Freiburg
Curated by ChEMBL
Albert-Ludwigs-University of Freiburg
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibitory activity against squalene hopene cyclase from Alicyclobacillus acidocaldariusMore data for this Ligand-Target Pair
Affinity DataIC50: 13.5nMAssay Description:Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of oxidosqualene cyclaseMore data for this Ligand-Target Pair
Affinity DataIC50: 13.5nMAssay Description:Inhibition of oxidosqualene cyclaseMore data for this Ligand-Target Pair