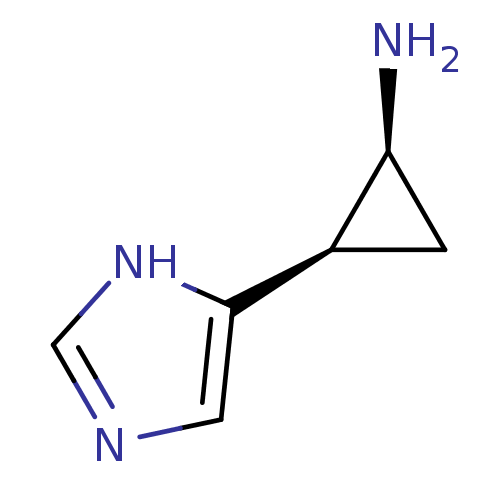
BDBM50127606 2-(1H-Imidazol-4-yl)-cyclopropylamine::CHEMBL294061
SMILES N[C@H]1C[C@H]1c1cnc[nH]1
InChI Key InChIKey=OWWNABDDYQLERE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50127606
Found 5 hits for monomerid = 50127606
Affinity DataEC50: >0.000100nMAssay Description:Inhibition of human Histamine H3 receptor using [3H]N-alpha-methyl histamineMore data for this Ligand-Target Pair
Affinity DataEC50: >0.000100nMAssay Description:Inhibition of human Histamine H1 receptor using [3H]pyrilamineMore data for this Ligand-Target Pair
Affinity DataEC50: >0.000100nMAssay Description:Inhibition of human Histamine H2 receptor using [3H]tiotidineMore data for this Ligand-Target Pair
Affinity DataEC50: >0.000100nMAssay Description:Inhibition of human Histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.38E+3nMAssay Description:Affinity against rat Histamine H3 receptor using [3H]N-alpha-methyl histamineMore data for this Ligand-Target Pair