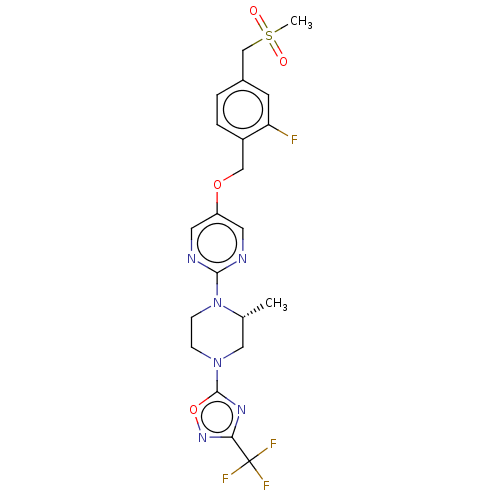
BDBM50103543 CHEMBL3358006
SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F
InChI Key InChIKey=HXNOCBIFNWVJLT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50103543
Found 12 hits for monomerid = 50103543
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of NaV1.5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CaV1.2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibition of IKs channel (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by patch clamp based electrophysiology methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin) by high throughput fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin) by high throughput fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin) by high throughput fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin) by high throughput fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) by high throughput fluorescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 68nMAssay Description:Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assayMore data for this Ligand-Target Pair