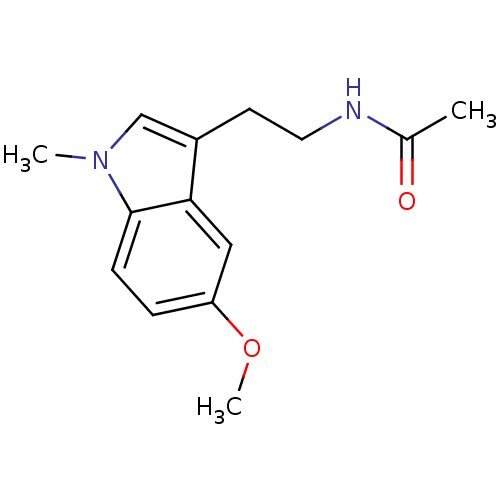
BDBM50066960 CHEMBL33700::N-(2-(5-methoxy-1-methyl-1H-indol-3-yl)ethyl)acetamide::N-[2-(5-Methoxy-1-methyl-1H-indol-3-yl)-ethyl]-acetamide
SMILES COc1ccc2n(C)cc(CCNC(C)=O)c2c1
InChI Key InChIKey=GSMWHFKFNGUYRN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50066960
Found 10 hits for monomerid = 50066960
Affinity DataIC50: 2.82nMAssay Description:Displacement of [125I]iodomelatonin from MT3/QR2 melatonin binding site expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of QR2 (unknown origin) using MTT and NMeH as substratesMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:inhibitory concentration against Melatonin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Binding Affinity (pKi) towards human Melatonin receptor type 1BMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Binding Affinity (pKi) towards human Melatonin receptor type 1AMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of 2-[125I]iodomelatonin binding to human Melatonin receptor type 1A expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Inhibition of 2-[125I]iodomelatonin binding to Melatonin receptor 3 (MT3) of Syrian hamster brain membraneMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Inhibition of 2-[125I]iodomelatonin binding to human Melatonin receptor type 1B expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.70nMAssay Description:Binding affinity for Melatonin receptor using 2-[125I]iodomelatonin as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 254nMAssay Description:Binding affinity towards melatonin receptor was determined using 2-[125I]iodomelatonin as radioligand in chick brain membranesMore data for this Ligand-Target Pair