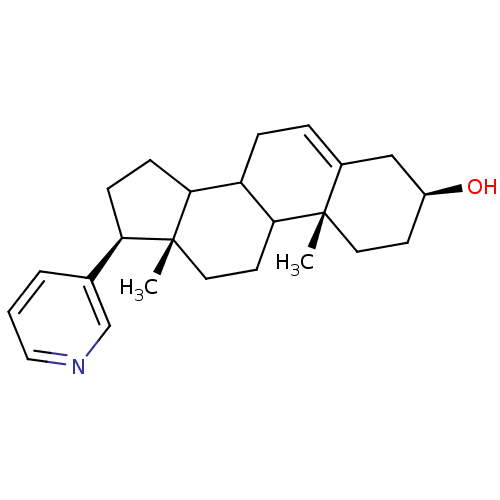
BDBM50031671 (3S,10R,13S,17S)-10,13-Dimethyl-17-pyridin-3-yl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol::CHEMBL82926
SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC[C@@H]2c1cccnc1
InChI Key InChIKey=MXMFCEUTOXUVHB-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50031671
Found 3 hits for monomerid = 50031671
Affinity DataIC50: 23nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of human progesterone 17-alpha-hydroxylase.More data for this Ligand-Target Pair