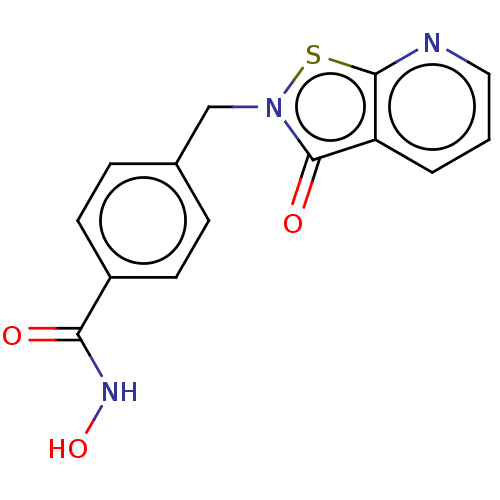
BDBM402284 (N-hydroxy-4-((3-oxoisothiazolo[5,4-b]pyridin-2(3H)-yl)methyl)benzamide)::US10011611, RBC-3001-A
SMILES ONC(=O)c1ccc(Cn2sc3ncccc3c2=O)cc1
InChI Key InChIKey=VLJAVCKTTLIBDT-UHFFFAOYSA-N
Data 11 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 402284
Found 11 hits for monomerid = 402284
Affinity DataIC50: 175nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 [395-489](Human)
Reaction Biology
US Patent
Reaction Biology
US Patent
Affinity DataIC50: 1.31E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.31E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 508nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.958nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.89E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 27.1nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.26E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.84E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair