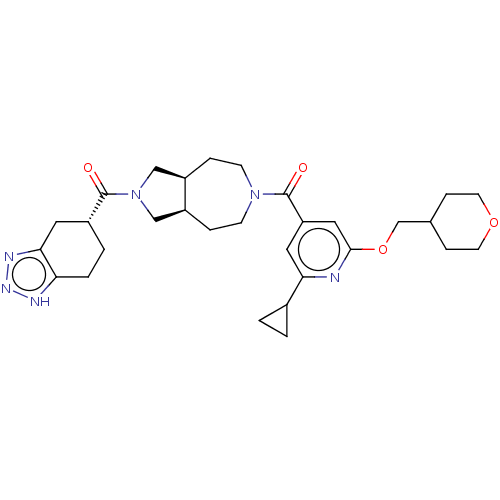
BDBM352364 US11098048, Example 2.21::US9802944, Example 2.21
SMILES O=C([C@@H]1CCc2[nH]nnc2C1)N1C[C@H]2CCN(CC[C@H]2C1)C(=O)c1cc(OCC2CCOCC2)nc(c1)C1CC1
InChI Key InChIKey=RQZOUUKHWSODHZ-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 352364
Found 2 hits for monomerid = 352364
Affinity DataIC50: 6nMAssay Description:ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Assay working solutions were made as follows:Assay buffer (50 mM Tris-HCl, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2), 1 mM MgCl2, 0.01% Triton-X-100, pH 8.0...More data for this Ligand-Target Pair