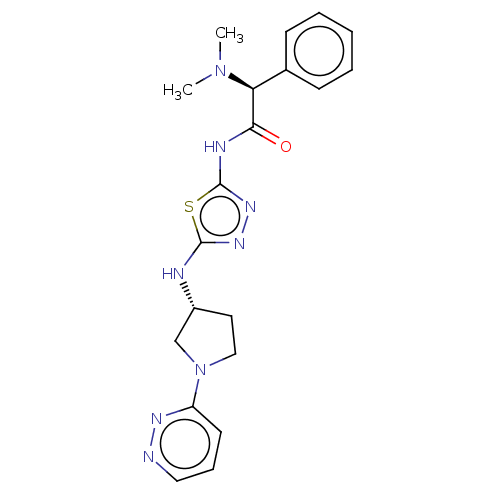
BDBM278398 (2S)-2-(Dimethylamino)-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-ylpyrrolidin-3-yl]amino]-1,3,4-thiadiazol-2-yl]acetamide and (2R)-2-(dimethylamino)-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-ylpyrrolidin-3-yl]amino]-1,3,4-thiadiazol-2-yl]acetamide::US10040789, Example 1(a)::US10040789, Example 1(b)
SMILES CN(C)[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1
InChI Key InChIKey=DFZKJNIPSCUEPU-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 278398
Found 2 hits for monomerid = 278398
Affinity DataIC50: 1.72E+3nMpH: 7.8 T: 2°CAssay Description:A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t...More data for this Ligand-Target Pair
Affinity DataIC50: 82.6nMpH: 7.8 T: 2°CAssay Description:A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t...More data for this Ligand-Target Pair