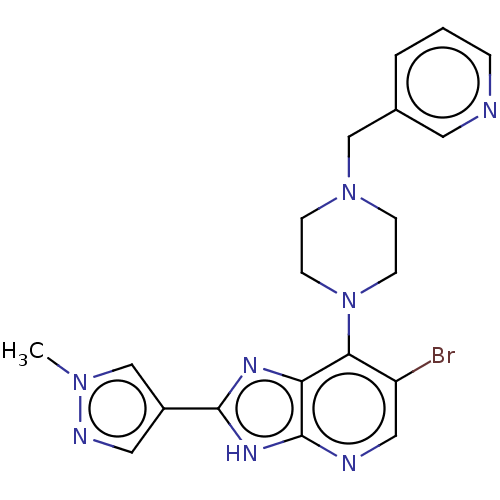
BDBM247367 US9447092, Comparator 1, Example 56
SMILES Cn1cc(cn1)-c1nc2c(N3CCN(Cc4cccnc4)CC3)c(Br)cnc2[nH]1
InChI Key InChIKey=KLCAJPNNJWCSJD-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 247367
Found 8 hits for monomerid = 247367
Affinity DataIC50: 32nMAssay Description:Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Cancer Research Technology
US Patent
Cancer Research Technology
US Patent
Affinity DataIC50: 5.50E+3nMAssay Description:All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair