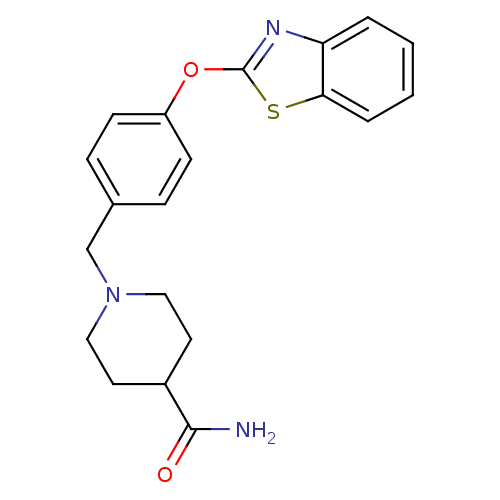
BDBM24233 1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}piperidine-4-carboxamide::Benzthiazole compound, 33h
SMILES NC(=O)C1CCN(Cc2ccc(Oc3nc4ccccc4s3)cc2)CC1
InChI Key InChIKey=MPIXODNJTUZDGF-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 24233
Found 3 hits for monomerid = 24233
Affinity DataIC50: 17nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of dofetilide binding to human ERG by patch clamp assayMore data for this Ligand-Target Pair