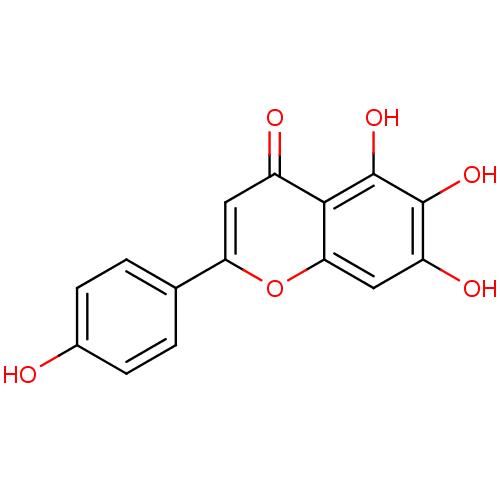
BDBM23411 5,6,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one::Scutel-larei::Scutellarein::Scutellarein (2)::Scutellarein (28)::US10252984, Table 2.2
SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)c(O)c(O)cc2o1
InChI Key InChIKey=JVXZRQGOGOXCEC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 29 hits for monomerid = 23411
Found 29 hits for monomerid = 23411
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant human N-terminal His6/SUMO-tagged PKM1 expressed in Escherichia coli BL21 using PEP as substrate incubated for 30 mins in p...More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 2B1(Human)
Soochow University
Curated by ChEMBL
Soochow University
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of human OATP2B1 expressed in Flp-In-CHO cells assessed as inhibition of OATP2B1 mediated DBF uptake using DBF as fluorescent substrate in...More data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibition of N-terminal full length GST-tagged SARS-CoV-2 3CLpro expressed in Escherichia coli BL21 (DE3) by using MCA-AVLQSGFR-Lys(Dnp)-Lys-NH as f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of PKM2 (unknown origin) Lys207, Hie78, Asp177, Asn75, Thr129, Gly128, Arg73 residuesMore data for this Ligand-Target Pair
Affinity DataIC50: 3.02E+3nMAssay Description:This is a review article. Please point to the original journal.More data for this Ligand-Target Pair
Affinity DataIC50: 3.82E+4nMAssay Description:Inhibition of human liver FBP1 incubated for 5 mins by fluorescence methodMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase NSD2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.65E+3nMAssay Description:Inhibition of NSD2 (unknown origin) preincubated for 15 mins followed by addition of [3H]-SAM as substrate and peptide solution after 240 mins cold S...More data for this Ligand-Target Pair
Affinity DataIC50: 2.92E+3nMAssay Description:Inhibition of MLL1 (unknown origin) preincubated for 15 mins followed by addition of substrate measured after 1 hr by AlphaLISA assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase SMYD3(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.82E+4nMAssay Description:Inhibition of SMYD3 (unknown origin) preincubated for 15 mins followed by addition of [3H]-SAM as substrate and peptide solution after 240 mins cold ...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH1(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 8.77E+3nMAssay Description:Inhibition of Ezh1 (unknown origin) preincubated for 15 mins followed by addition of substrate measured after 1 hr by AlphaLISA assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase SETD7(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.96E+3nMAssay Description:Inhibition of human SET7 overexpressed in Escherichia coli BL21 (DE3) cells preincubated for 15 mins followed by addition of SAM as substrate and bio...More data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
Universidad De Buenos Aires
Curated by ChEMBL
Universidad De Buenos Aires
Curated by ChEMBL
Affinity DataIC50: 4.89E+4nMAssay Description:Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assayMore data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
Universidad De Buenos Aires
Curated by ChEMBL
Universidad De Buenos Aires
Curated by ChEMBL
Affinity DataIC50: 5.05E+4nMAssay Description:Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase S(Human)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of recombinant human PTP-sigma (residues 1367 to 1948) using para-nitrophenylphosphate as substrate for 60 mins by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of recombinant human N-terminal His6/SUMO-tagged PKM2 expressed in Escherichia coli BL21 using PEP as substrate incubated for 30 mins in p...More data for this Ligand-Target Pair
Affinity DataIC50: 9.64E+3nMpH: 6.0 T: 2°CAssay Description:The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant FLT3 (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibition of human Neu2 assessed as MuNANA substrate hydrolysis in presence of 0.1% Triton X-100 by discontinuous fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of cow milk xanthine oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of AChE in rat cortex using acetylthiocholine iodide as substrate after 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+6nMAssay Description:Inhibition of SSADH (unknown origin)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Mouse)
Institute of Chinese Materia Medica
Curated by ChEMBL
Institute of Chinese Materia Medica
Curated by ChEMBL
Affinity DataEC50: 1.64E+4nMAssay Description:Agonist activity at mouse PPARgamma expressed in HEK293 cells co-expressing with Gal4 reporter vector after 24 hrs by dual-luciferase reporter assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:For rat AChE or BuChE inhibition assays, a reaction mixture (100 μL) containing acetylthiocholine iodide (1 mmol/L, 30 μL) (J&K Scientific)...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:For rat AChE or BuChE inhibition assays, a reaction mixture (100 μL) containing acetylthiocholine iodide (1 mmol/L, 30 μL) (J&K Scientific)...More data for this Ligand-Target Pair
Affinity DataIC50: 3.85E+3nMAssay Description:The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The...More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufinMore data for this Ligand-Target Pair
Affinity DataKi: 1.64E+3nMAssay Description:Inhibition of CYP1A1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufinMore data for this Ligand-Target Pair
Affinity DataKi: 4.13E+4nMAssay Description:Drug metabolism assessed as human recombinant UGT1A1-mediated formation of scutellarein-7-O-glucuronide after 25 mins by HPLC/UV analysisMore data for this Ligand-Target Pair