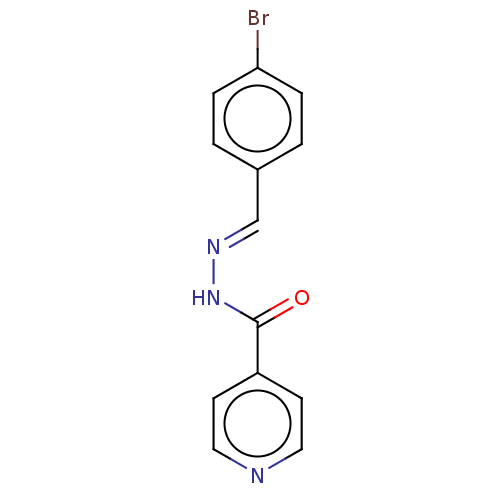
BDBM223072 (E)-N'-(4'-Bromobenzylidene)isonicotinohydrazide (3b)
SMILES Brc1ccc(\C=N\NC(=O)c2ccncc2)cc1
InChI Key InChIKey=WJDGJNGJDIMLRB-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 223072
Found 4 hits for monomerid = 223072
Affinity DataIC50: 230nMpH: 7.4 T: 2°CAssay Description:The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock...More data for this Ligand-Target Pair
Affinity DataIC50: 7.13E+3nMpH: 7.4 T: 2°CAssay Description:The enzymatic assay of both human and rat ecto-5'-nucleotidase were performed with slight modifications in the previously described method. The stock...More data for this Ligand-Target Pair
Affinity DataIC50: 4.13E+3nMpH: 9.8 T: 2°CAssay Description:The assay conditions were optimized with some changes in formerly reported spectrophotometric method. The composition of assay buffer of pH 9.8 was a...More data for this Ligand-Target Pair
Affinity DataIC50: 9.72E+3nMpH: 9.8 T: 2°CAssay Description:The assay conditions were optimized with some changes in formerly reported spectrophotometric method. The composition of assay buffer of pH 9.8 was a...More data for this Ligand-Target Pair