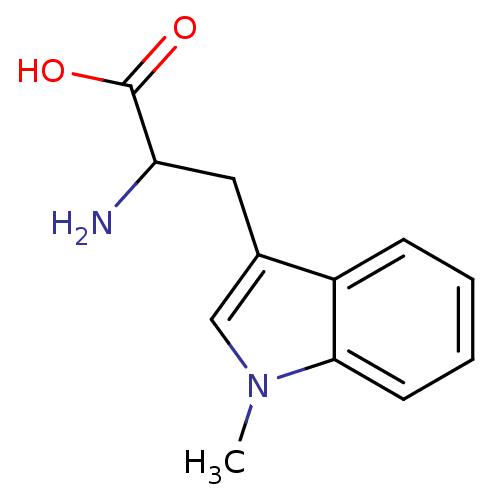
BDBM21973 1-Methyltryptophan, 1::2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid
SMILES Cn1cc(CC(N)C(O)=O)c2ccccc12
InChI Key InChIKey=ZADWXFSZEAPBJS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 21973
Found 10 hits for monomerid = 21973
Affinity DataIC50: 3.28E+5nMAssay Description:Inhibition of human recombinant indoleamine-2,3-dioxygenase expressed in Escherichia coli BL21 using L-tryptophan as substrate after 30 mins by micro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+4nMAssay Description:Inhibition of IDO1 in human IFN-gamma stimulated human HeLa cells assessed as inhibition of kynurenine production using L-tryptophan substrate incuba...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+5nMAssay Description:Inhibition of human IDO1 assessed as reduction in kynurenine productionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.67E+5nMAssay Description:Inhibition of human IDO1 expressed in African green monkey COS1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+5nMAssay Description:Inhibition of human recombinant IDO1 expressed in Escherichia coli BL21 incubated for 6 hrsMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+4nMAssay Description:Inhibition of human recombinant IDO using L-tryptophan as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+4nMAssay Description:Competitive inhibition of indoleamine-2,3-dioxygenaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+4nMAssay Description:Inhibition of IDO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 6.20E+4nM ΔG°: -5.97kcal/molepH: 6.5 T: 2°CAssay Description:The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as...More data for this Ligand-Target Pair