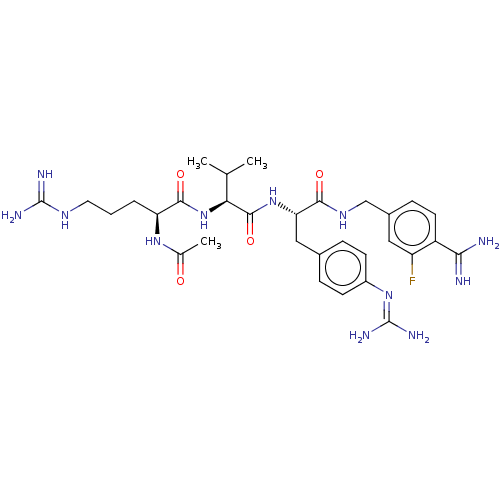
BDBM207839 US9266828, B
SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(-[#6](-[#7])=[#7])c(F)c1
InChI Key InChIKey=JBZBMYMLCWBKFW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 207839
Found 2 hits for monomerid = 207839
Affinity DataIC50: 3.70nMpH: 7.5 T: 2°CAssay Description:Furin activity was measured in triplicate in wells of a 96-well plate in 0.2 ml 50 mM HEPES, pH 7.5, containing 1 mM CaCl2, 0.005% Brij-35 and 20% gl...More data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of human furin using Pyr-RTKR-AMC as substrate preincubated for 30 mins followed by substrate addition and measured by fluorescence based ...More data for this Ligand-Target Pair