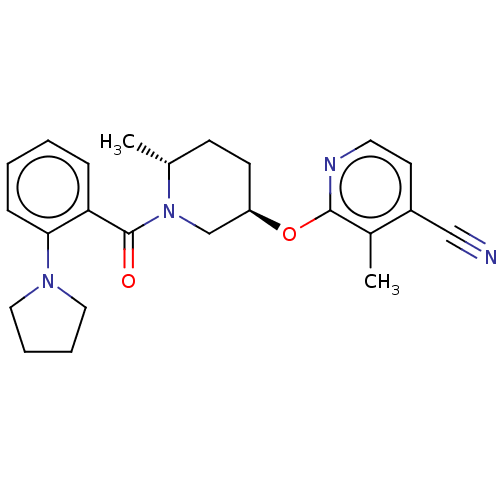
BDBM205120 3-methyl-2-({(3r,6r)-6- methyl-1-[(2-pyrrolidin-1- ylphenyl)carbonyl]piperidin-3- yl}oxy)pyridine-4-carbonitrile::US9546152, example 29
SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1N1CCCC1)Oc1nccc(C#N)c1C
InChI Key InChIKey=SUQPHIUGBSAEAH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 205120
Found 8 hits for monomerid = 205120
Affinity DataIC50: 29nMAssay Description:The utility of the compounds in accordance with the present invention as orexin receptor OX1R and/or OX2R antagonists may be readily determined witho...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:The utility of the compounds in accordance with the present invention as orexin receptor OX1R and/or OX2R antagonists may be readily determined witho...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Antagonist activity at human OX2R expressed in CHOK1 cells assessed as reduction in orexin A-induced calcium flux preincubated followed by orexin A a...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Antagonist activity at human OX1R expressed in CHOK1 cells assessed as reduction in orexin A-induced calcium flux preincubated followed by orexin A a...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which the inhibition constant (Ki) is determ...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp...More data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which the inhibition constant (Ki) is determ...More data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C...More data for this Ligand-Target Pair