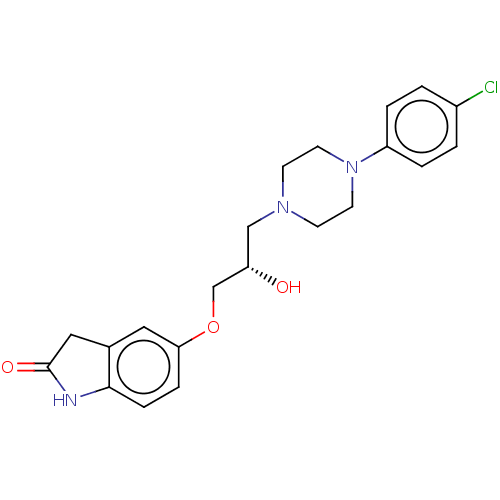
BDBM168624 US9079852, Table F, Compound 9
SMILES O[C@H](COc1ccc2NC(=O)Cc2c1)CN1CCN(CC1)c1ccc(Cl)cc1
InChI Key InChIKey=RUXMKHKIUOUGFV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 168624
Found 4 hits for monomerid = 168624
Affinity DataIC50: 100nMAssay Description:Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair