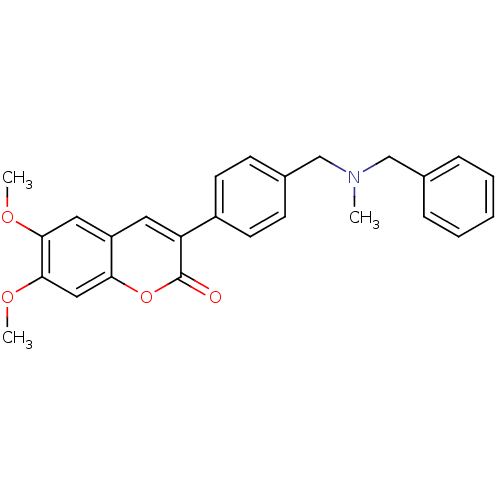
BDBM10949 3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dimethoxy-2H-2-chromenone::3-(4-{[benzyl(methyl)amino]methyl}phenyl)-6,7-dimethoxy-2H-chromen-2-one::AP2238::CHEMBL75121
SMILES COc1cc2cc(-c3ccc(CN(C)Cc4ccccc4)cc3)c(=O)oc2cc1OC
InChI Key InChIKey=KXMCSAUVAHKCOR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 10949
Found 15 hits for monomerid = 10949
Affinity DataIC50: 4.89E+4nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob...More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of human recombinant AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 4.89E+4nMAssay Description:Inhibition of human recombinant BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.89E+4nMAssay Description:Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.89E+4nMAssay Description:Inhibition of BuChEMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of human AchEMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of human recombinant AChE by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of human AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 1.24E+4nMAssay Description:Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to ...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured up to 180...More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of acetylcholinesterase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 21.7nM ΔG°: -10.9kcal/mole IC50: 44.5nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob...More data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Inhibition of recombinant human AChE assessed as hydrolysis of acetylthiocholine by Ellman's methodMore data for this Ligand-Target Pair