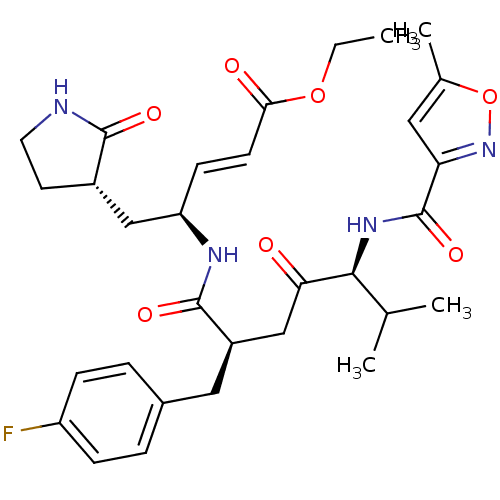
BDBM11243 AG7088::CHEMBL20210::US11859014, Compound rupintrivir::cmdc.202100576, 24f::ethyl (2E,4S)-4-[((2R,5S)-2-(4-fluorobenzyl)-6-methyl-5-{[(5-methylisoxazol-3-yl)carbonyl]amino}-4-oxoheptanoyl)amino]-5-[(3S)-2-oxopyrrolidin-3-yl]pent-2-enoate::ethyl (2E,4S)-4-[(2R,5S)-2-[(4-fluorophenyl)methyl]-6-methyl-5-[(5-methyl-1,2-oxazol-3-yl)formamido]-4-oxoheptanamido]-5-[(3S)-2-oxopyrrolidin-3-yl]pent-2-enoate::med.21724, Compound 29
SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@@H](CC(=O)[C@@H](NC(=O)c1cc(C)on1)C(C)C)Cc1ccc(F)cc1
InChI Key InChIKey=CAYJBRBGZBCZKO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 11243
Found 12 hits for monomerid = 11243
Affinity DataIC50: 1.00E+5nMpH: 7.0 T: 2°CAssay Description:The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Reversible inhibitory activity against ProteaseMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Increased percentage of formazan production in drug treated virus infected cells to equal 50% control drug free uninfected cells on serotype 14More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+5nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Please point to the patents.More data for this Ligand-Target Pair
Affinity DataIC50: 2.36E+4nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of SARS-CoV-2 MProMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase alpha(Human)
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant full length GST tagged P14KIII alpha (residues 1 to 854) incubated for 10 mins by ADP-Glo kinase methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Human)
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant GST tagged P14KIII beta incubated for 10 mins by ADP-Glo kinase methodMore data for this Ligand-Target Pair
Affinity DataKi: 8.20E+3nMAssay Description:The ability of these compounds to inhibit the NoV, specifically Minerva virus protease catalytic Cys139 covalently (IC50 and Ki) was determined with ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair