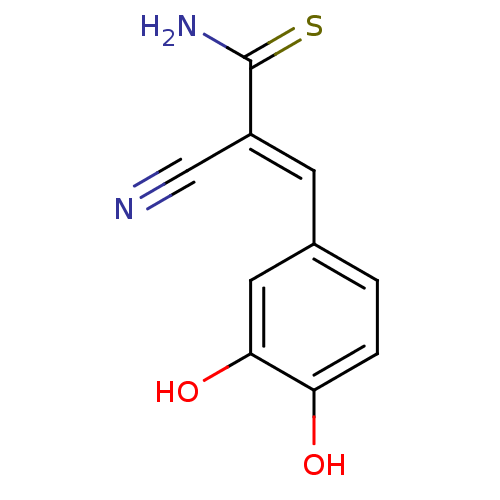
BDBM4363 (2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enethioamide::AG 213::CHEMBL77030::RG-50864::T7540 (Tyrphostin 47)::Tyrphostin A47::alpha-Cyano-(3,4-dihydroxy)thiocinnamide::benzylidenemalononitrile (BMN) deriv. 47
SMILES NC(=S)C(=C\c1ccc(O)c(O)c1)\C#N
InChI Key InChIKey=ZGHQGWOETPXKLY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 4363
Found 14 hits for monomerid = 4363
Affinity DataIC50: 2.90E+3nMpH: 7.5 T: 2°CAssay Description:The full-length cFMS cytoplasmic domain (FMS.538-972.6His) or chimera was incubated with compound in reaction buffer. Control reactions were run in e...More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor [538-678,753-922](Human)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 3.30E+3nMpH: 7.5 T: 2°CAssay Description:The full-length cFMS cytoplasmic domain (FMS.538-972.6His) or chimera was incubated with compound in reaction buffer. Control reactions were run in e...More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor [538-678,753-922](Human)
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 3.00E+3nMpH: 7.5 T: 2°CAssay Description:The full-length cFMS cytoplasmic domain (FMS.538-972.6His) or chimera was incubated with compound in reaction buffer. Control reactions were run in e...More data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2/Receptor protein-tyrosine kinase(Human)
Duke University Medical Center
Duke University Medical Center
Affinity DataIC50: 3.00E+3nMT: 2°CAssay Description:In order to eliminated fluorescence interference, chemicals were subjected to a secondary screening using colorimetric BP incorporation assay. TGase...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of EGFR in human A431 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+4nMAssay Description:Inhibitory activity against dynamin1 GTPase expressed in sheep brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+3nMAssay Description:Inhibition of human EGFR tyrosine kinase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of human HER2 tyrosine kinase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of recombinant SykMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of EGF-dependent proliferation of human and guinea pig keratinocytes; range 7-15 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human recombinant Tdp1 assessed as conversion of 14-mer 5'-32P-labeled 3'-phosphotyrosyl DNA substrate N14Y to 14-mer 5'-32P-labeled 3'...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+5nMAssay Description:Inhibition of IRKMore data for this Ligand-Target Pair
Affinity DataKi: 850nM IC50: 2.40E+3nMAssay Description:The activity of EGFR, preactivated with EGF, is measured by its ability to transfer terminal phosphate from [gamma-32P]ATP to poly(GAT) substrate.More data for this Ligand-Target Pair