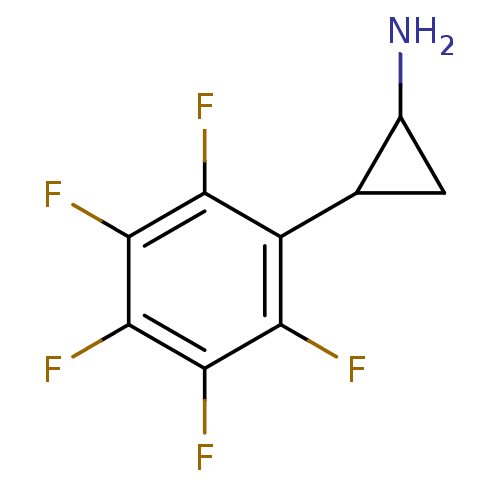
BDBM50346865 2-PFPA::CHEMBL1797642
SMILES NC1CC1c1c(F)c(F)c(F)c(F)c1F
InChI Key InChIKey=NZZZWLLBMMVQQY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50346865
Found 10 hits for monomerid = 50346865
Affinity DataIC50: 8.90E+3nMAssay Description:The kinetic inhibition parameters of LSD1 demethylase inhibition were obtained using the peroxidase-coupled reaction method.More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of LSD1More data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+3nMAssay Description:Inhibition of hexahistidine-tagged LSD1 (unknown origin) (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptid...More data for this Ligand-Target Pair
Affinity DataKi: 8.30E+3nMAssay Description:Inhibition of human MAO-B expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled methodMore data for this Ligand-Target Pair
Affinity DataKi: 8.30E+3nMAssay Description:Inhibition of human recombinant MAO-B using tyramine as substrate assessed as inhibition constant by peroxidase-coupled reaction assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Inhibition of hexahistidine-tagged LSD1 (unknown origin) (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptid...More data for this Ligand-Target Pair
Affinity DataKi: 2.70E+4nMAssay Description:Inhibition of human recombinant MAO-A using tyramine as substrate assessed as inhibition constant by peroxidase-coupled reaction assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.70E+5nMAssay Description:Inhibition of human MAO-A expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled methodMore data for this Ligand-Target Pair