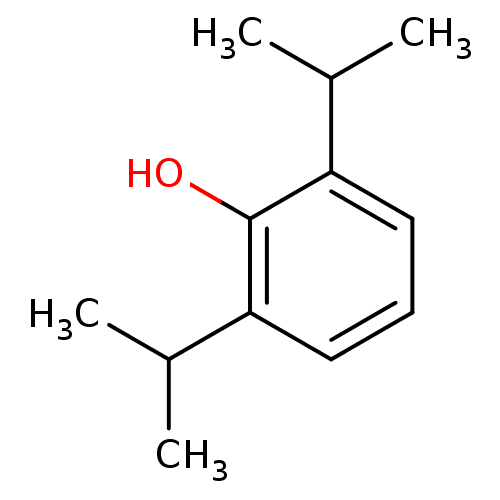
BDBM50058046 CHEMBL526::propofol
SMILES CC(C)c1cccc(C(C)C)c1O
InChI Key InChIKey=OLBCVFGFOZPWHH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 50058046
Found 16 hits for monomerid = 50058046
Affinity DataEC50: 4.40E+4nMAssay Description:Direct activation of chloride current in Xenopus laevis oocytes expressing human alpha-1-beta-1-gamma-2 GABA-A receptor subunitsMore data for this Ligand-Target Pair
Affinity DataEC50: 8.00E+3nMAssay Description:Modulation of GABA-induced chloride currents in Xenopus laevis oocytes expressing human alpha-1-beta-1-gamma-2 GABA-A receptor subunitsMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2(Human)
Organon Research and Development Group
Curated by ChEMBL
Organon Research and Development Group
Curated by ChEMBL
Affinity DataEC50: 4.10E+3nMAssay Description:GABA-modulatory action compound was evaluated on Oocytes expressing recombinant human alpha-1-beta-3-gamma-2L GABA A receptor at concentrations of >=...More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2(Human)
Organon Research and Development Group
Curated by ChEMBL
Organon Research and Development Group
Curated by ChEMBL
Affinity DataEC50: 5.00E+4nMAssay Description:GABA-mimetic action on Oocytes expressing human alpha-1-beta-3-gamma-2L GABA-A receptor subunits at >=100 uMMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2(Human)
University of Sassari
Curated by ChEMBL
University of Sassari
Curated by ChEMBL
Affinity DataEC50: 1.12E+4nMAssay Description:Effective concentration against GABA-evoked chloride currents mediated by human Gamma-aminobutyric acid GABA-A receptor alpha2-beta2-gamma2L expresse...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+4nMAssay Description:Irreversible inhibition of fatty acid amide hydrolase; range=. 5-6 nMMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of rat FAAHMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2(Human)
University of Chicago Medical Center
Curated by ChEMBL
University of Chicago Medical Center
Curated by ChEMBL
Affinity DataEC50: 1.00E+4nMAssay Description:Direct activation of human Gamma-aminobutyric acid A receptor alpha-1-beta-2-gamma-2More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2(Human)
University of Chicago Medical Center
Curated by ChEMBL
University of Chicago Medical Center
Curated by ChEMBL
Affinity DataEC50: 2.00E+3nMAssay Description:Potentiation of GABA responses at human Gamma-aminobutyric acid A receptor alpha-1-beta-2-gamma-2More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2(Human)
Organon Research and Development Group
Curated by ChEMBL
Organon Research and Development Group
Curated by ChEMBL
Affinity DataEC50: 5.40E+3nMAssay Description:Positive allosteric modulation of GABAA alpha1beta3gamma2L in HEK cell plasma membrane assessed as increase in [3H]muscimol binding measured after 10...More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2(Human)
Organon Research and Development Group
Curated by ChEMBL
Organon Research and Development Group
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of [3H]azietomidate labelling of GABAA alpha1beta3gamma2L (unknown origin) expressed in Xenopus laevis oocytes assessed as reduction in ph...More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2(Human)
Organon Research and Development Group
Curated by ChEMBL
Organon Research and Development Group
Curated by ChEMBL
Affinity DataIC50: 4.40E+4nMAssay Description:Inhibition of [3H]R-mTFD-MPAB labelling of GABAA alpha1beta3gamma2L (unknown origin) expressed in Xenopus laevis oocytes assessed as reduction in pho...More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2(Human)
Organon Research and Development Group
Curated by ChEMBL
Organon Research and Development Group
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of [3H]pTFD-di-iPr-BnOH labelling of GABAA alpha1beta3gamma2L (unknown origin) expressed in Xenopus laevis oocytes assessed as reduction i...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
The First Affiliated Hospital of Zhengzhou University
Curated by ChEMBL
The First Affiliated Hospital of Zhengzhou University
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:Inhibition of TRPV1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.95E+4nMAssay Description:Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrateMore data for this Ligand-Target Pair
Affinity DataKi: 9.89E+4nMAssay Description:Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrateMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)