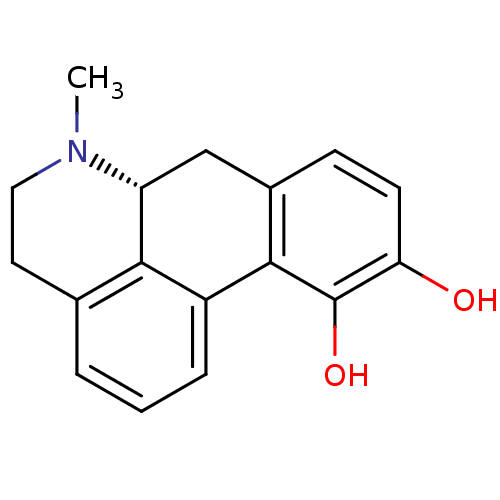
BDBM29644 (6aR)-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-10,11-diol;hydrate;hydrochloride::APOMORPHINE HYDROCHLORIDE::APOMORPHINE HYDROCHLORIDE HEMIHYDRATE::MLS000069811::SMR000059235::US20240166639, Example Apomorphine::cid_6852399
SMILES CN1CCc2cccc-3c2[C@H]1Cc1ccc(O)c(O)c-31
InChI Key InChIKey=VMWNQDUVQKEIOC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 29 hits for monomerid = 29644
Found 29 hits for monomerid = 29644
Affinity DataEC50: 1.69E+4nMAssay Description:Broad Institute: MLPCN maternal gene expression Project ID: 2024 Keywords: Zinc finger, C. elegans, maternal gene expression, RNA-protein interac...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Concentration necessary to achieve half maximal inhibition of [3H](+)-23amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene binding to dopamine recepto...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Compound was tested for inhibitory binding activity against dopamine receptor in rat striatal membranes using [3H]HAL as the radioligand.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rat)
Istituto Superiore Di Sanit£
Curated by ChEMBL
Istituto Superiore Di Sanit£
Curated by ChEMBL
Affinity DataIC50: 302nMAssay Description:Ability to inhibit the specific binding of [3H]- dihydromorphine to opiate receptors in rat brain membrane preparation by 50%More data for this Ligand-Target Pair
Affinity DataIC50: 182nMAssay Description:Concentration necessary to achieve half maximal inhibition of [3H]spiperone binding dopamine receptor at 1 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 87nMAssay Description:Displacement of [3H]spiperone from rat dopamine D2 receptor in brain striatal membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:The IC50 value was reported as apparent, since [3H]NCA was purported to be irreversible. Result indicates the mean of two separate experiments, each ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+5nMAssay Description:Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t...More data for this Ligand-Target Pair
Affinity DataEC50: 1.89E+4nMpH: 7.4 T: 2°CAssay Description:The HTS assay was conducted in 384-well microplates in a total assay volume per well of 10.1 microliters (5 microliters of bead mixture, 0.1 microlit...More data for this Ligand-Target Pair
TargetDNA dC->dU-editing enzyme APOBEC-3G(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.62E+4nMT: 2°CAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 4.42E+3nMAssay Description:Broad Institute: MLPCN maternal gene expression Project ID: 2024 Keywords: Zinc finger, C. elegans, maternal gene expression, RNA-protein interac...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1(Human)
Emory University
Curated by PubChem BioAssay
Emory University
Curated by PubChem BioAssay
Affinity DataIC50: 4.38E+3nMAssay Description:NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Nikolovska-Coleska, Univer...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 4 gamma 1(Human)
Emory University
Curated by PubChem BioAssay
Emory University
Curated by PubChem BioAssay
Affinity DataIC50: 6.40E+4nMAssay Description:Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation NIH Molecular Libraries Screening Centers Network [MLSC...More data for this Ligand-Target Pair
Affinity DataEC50: 4.23E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: NIH 1X01 MH079850-01 HTS to identify small molecule regulators of Bcl-2 family protein int...More data for this Ligand-Target Pair
Affinity DataEC50: 1.90E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: NIH 1X01 MH079850-01 HTS to identify small molecule regulators of Bcl-2 family protein int...More data for this Ligand-Target Pair
TargetMannose-6-phosphate isomerase(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.46E+4nMpH: 7.4 T: 2°CAssay Description:The purpose of this assay is to identify non-competititve inhibitors of human PMI. This is accomplished by using a G6PD- NADPH-coupled assay. In the ...More data for this Ligand-Target Pair
Affinity DataEC50: 4.64E+4nMpH: 7.4 T: 2°CAssay Description:The multiplex is constructed by using beads for each protein target that have been labeled with varying intensities of red color, so that each assay ...More data for this Ligand-Target Pair
TargetRunt-related transcription factor 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 9.81E+3nMpH: 7.4 T: 2°CAssay Description:This assay is to identify inhibitors of the protein-protein interaction between the RUNX1 Runt domain and CBFbeta-SMMHC. This is accomplished by usin...More data for this Ligand-Target Pair
TargetRunt-related transcription factor 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.34E+4nMpH: 7.4 T: 2°CAssay Description:This assay is to identify inhibitors of the protein-protein interaction between the RUNX1 Runt domain and CBFbeta-SMMHC. This is accomplished by usin...More data for this Ligand-Target Pair
TargetRunt-related transcription factor 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.89E+4nMpH: 7.4 T: 2°CAssay Description:This assay is to identify inhibitors of the protein-protein interaction between the RUNX1 Runt domain and CBFbeta-SMMHC. This is accomplished by usin...More data for this Ligand-Target Pair
TargetRunt-related transcription factor 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 9.81E+3nMpH: 7.4 T: 2°CAssay Description:This assay is to use HTS to identify inhibitors of the protein-protein interaction between the RUNX1 Runt domain and CBFbeta-SMMHC, a potential thera...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rat)
LEUKOS BIOTECH, S.L.; FUNDACIÓ INSTITUT DE RECERCA CONTRA LA LEUCÈMIA JOSEP CARRERAS
US Patent
LEUKOS BIOTECH, S.L.; FUNDACIÓ INSTITUT DE RECERCA CONTRA LA LEUCÈMIA JOSEP CARRERAS
US Patent
Affinity DataKi: 6.92nMAssay Description:Serotonin 5-HT2A receptor competition binding experiments were carried out in a polypropilene 96-well plate. In each well was incubated 80 μg of...More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in SF9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in SF9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 42nMAssay Description:In vitro binding affinity towards cloned human Dopamine receptor D2A using [3H]- Raclopride as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Displacement of [3H]spiperone from rat dopamine D2 receptor in brain striatal membraneMore data for this Ligand-Target Pair
Affinity DataKi: 188nMAssay Description:In vitro binding affinity towards cloned rat 5-hydroxytryptamine 7 receptor using [3H]5-HT as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 296nMAssay Description:In vitro binding affinity towards cloned human 5-hydroxytryptamine 1A receptor expressed in Chinese hamster ovary (CHO) cells using [3H]8-OH-DPAT as ...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1B(Human)
LEUKOS BIOTECH, S.L.; FUNDACIÓ INSTITUT DE RECERCA CONTRA LA LEUCÈMIA JOSEP CARRERAS
US Patent
LEUKOS BIOTECH, S.L.; FUNDACIÓ INSTITUT DE RECERCA CONTRA LA LEUCÈMIA JOSEP CARRERAS
US Patent
Affinity DataKi: 1.76E+3nMAssay Description:Serotonin 5-HT1B receptor competition binding experiments were carried out in a polypropylene 96-well plate. In each well was incubated 5 μg of ...More data for this Ligand-Target Pair